Pfizer Covid Vaccine Phase 3

An accumulation the top Pfizer Covid Vaccine Phase 3 wallpapers and backgrounds readily available for download for free. Hopefully you enjoy our growing number of HD images to make use of as a background or home screen for your smartphone or computer. Please contact us if you want to publish a beautifull wallpaper on our site. Only the best wallpapers. Daily improvements of new, great, HD wallpapers for desktop and phones.
A ton of awesome Pfizer Covid Vaccine Phase 3 wallpapers to help download to get free. Additionally you can include along with discuss your preferred wallpapers. HD wallpapers and background photos
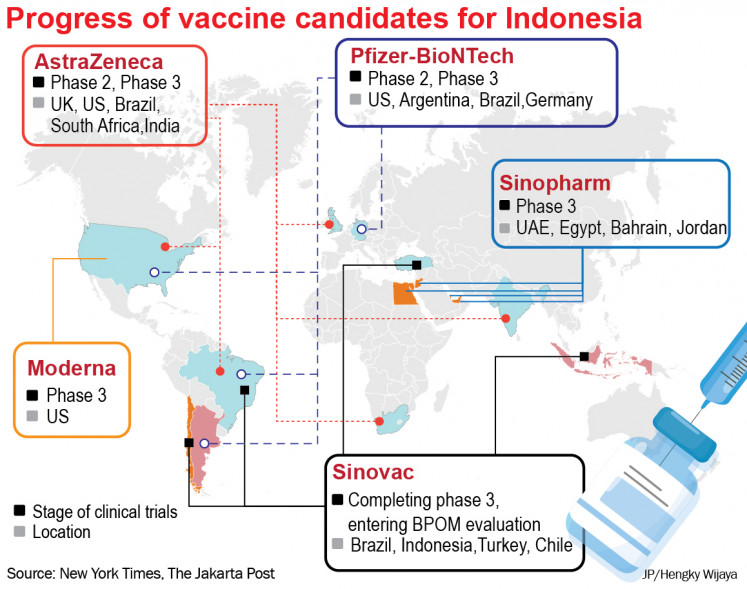
Ri Greenlights Adoption Of Major Covid 19 Vaccines Mon December 7 2020 The Jakarta Post
Pfizer Covid 19 Vaccine Candidate Over 90 Percent Effective Study Shows
Coronavirus Vaccine 90 Effective Say Pfizer And German Company Biontech News Dw 09 11 2020

Pfizer covid vaccine phase 3
Bnt162b2 the two dose messenger rna mrna vaccine from pfizer and biontech will undergo a full day meeting in which its associated phase 3 data from an ongoing multi national assessment will be scrutinized and weighed by a panel of experts with public input before being voted on as either indicative of a positive or negative benefit risk ratio for its prospective use as a preventive vaccine for confirmed covid 19. Pfizer and biontech announced that the ongoing phase 3 study for our covid 19 vaccine candidate has met all of its primary efficacy endpoints. Pfizer and the german biotech company biontech said on monday that they had had encouraging early results from a phase 3 clinical trial of their coronavirus vaccine. Pfizer and biontech conclude phase 3 study of covid 19 vaccine candidate meeting all primary efficacy endpoints wednesday november 18 2020 06 59am primary efficacy analysis demonstrates bnt162b2 to be 95 effective against covid 19 beginning 28 days after the first dose. The phase 3 trials of the pfizer biontech vaccine involved 42 000 people about half of whom got the experimental vaccine and the rest a placebo.
Related post:


Pfizer And Biontech Conclude Phase 3 Study Of Covid 19 Vaccine Candidate Nordic Life Science The Leading Nordic Life Science News Service

Pfizer Concludes Phase 3 Study Of Covid 19 Vaccine Candidate Says It S 95 Effective Oneindia News
Https Www Alabamapublichealth Gov Covid19 Assets Cov Pfizer Moderna Vaccine Trial Summary Pdf

Covid 19 Vaccine Update Pfizer Files For Emergency Use Covaxin Trials To Start In Metro Cities

Covid Vaccine Side Effects What It S Like In Pfizer Moderna Trials

Pfizer Biontech Nab Fast Track Tag Prep For Major Phase 3 Covid 19 Vax Test This Month Fiercebiotech

Pfizer Biontech And Moderna Begin Late Stage Covid 19 Vaccine Trials

Step Closer To Ending Health Crisis Pfizer Says Its Covid 19 Vaccine 90 Effective In Phase 3 Trial

Covid 19 Update Here S Where We Stand Now In The Race For A Vaccine Tif

Mtvt9ufi2ybgqm

Coronavirus Us Fda Backs Biontech Pfizer Covid Vaccine Data News Dw 08 12 2020

Covid 19 Vaccine Update Pfizer Announces 95 Efficacy Spain Authorises Phase 3 Trial Of Janssen S Candidate India News Hindustan Times

Uk Set For Vaccination Next Week While Eu Says More Time Is Needed Cgtn
Pfizer S Coronavirus Vaccine Requires 2 Shots 3 Weeks Apart Business Insider

Pfizer Biontech S Covid 19 Vaccine Is 95 Effective Final Analysis Shows Pmlive
:strip_icc():format(jpeg)/kly-media-production/medias/3324088/original/013682900_1608006442-Hoaks_Singapura_Lakukan_Vaksinasi_Covid-19_di_Bandara_Changi.jpg)
Cek Fakta Hoaks Singapura Lakukan Vaksinasi Covid 19 Di Bandara Changi Simak Penelusurannya Cek Fakta Liputan6 Com

6ojpfcbcraroxm
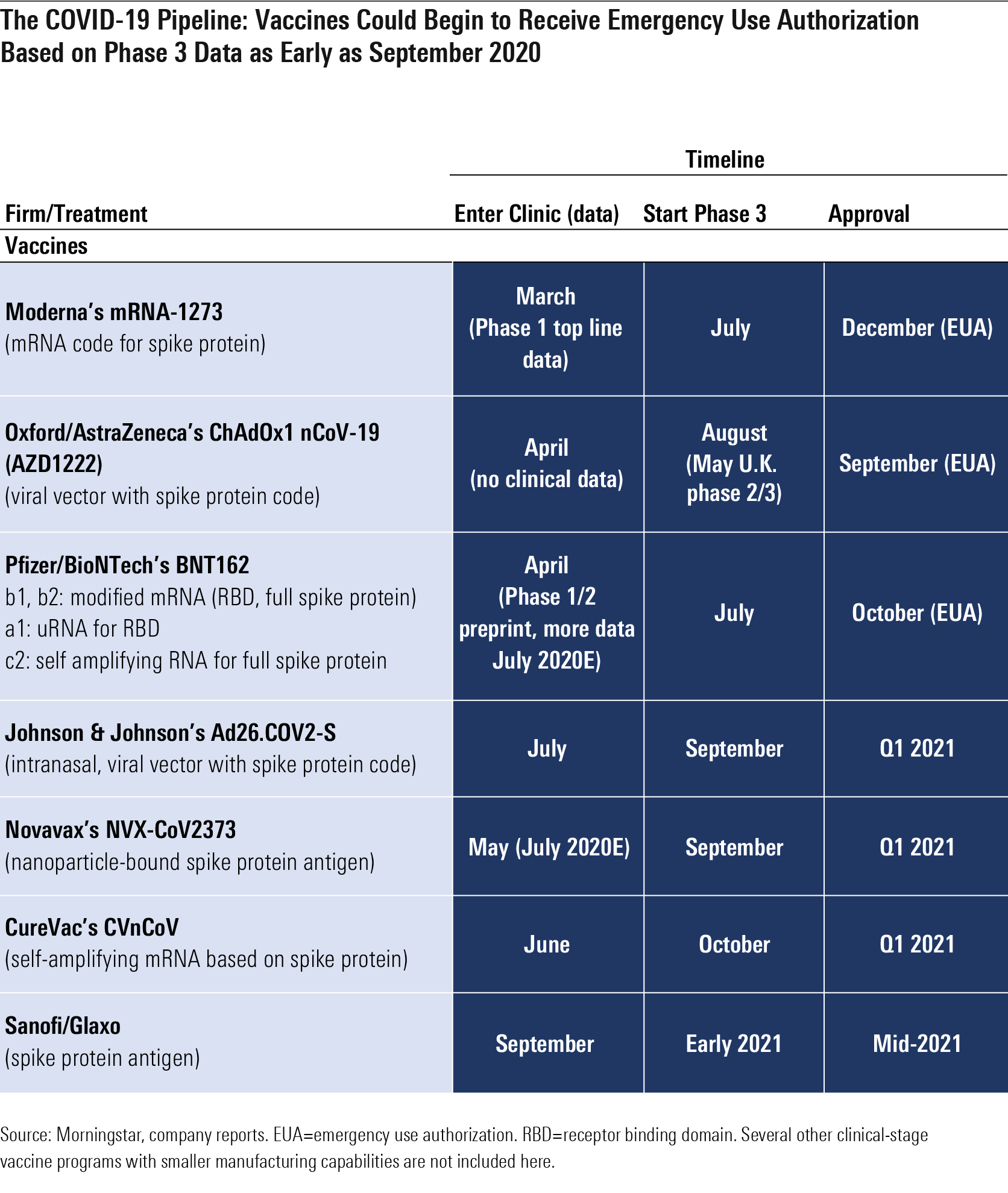
Timeline Accelerates For Covid 19 Drug Development Morningstar
That's all about Pfizer Covid Vaccine Phase 3, The phase 3 trials of the pfizer biontech vaccine involved 42 000 people about half of whom got the experimental vaccine and the rest a placebo. Pfizer and biontech conclude phase 3 study of covid 19 vaccine candidate meeting all primary efficacy endpoints wednesday november 18 2020 06 59am primary efficacy analysis demonstrates bnt162b2 to be 95 effective against covid 19 beginning 28 days after the first dose. Pfizer and the german biotech company biontech said on monday that they had had encouraging early results from a phase 3 clinical trial of their coronavirus vaccine. Pfizer and biontech announced that the ongoing phase 3 study for our covid 19 vaccine candidate has met all of its primary efficacy endpoints. Bnt162b2 the two dose messenger rna mrna vaccine from pfizer and biontech will undergo a full day meeting in which its associated phase 3 data from an ongoing multi national assessment will be scrutinized and weighed by a panel of experts with public input before being voted on as either indicative of a positive or negative benefit risk ratio for its prospective use as a preventive vaccine for confirmed covid 19.



