Fda Covid Vaccine Guidance

An accumulation the top Fda Covid Vaccine Guidance wallpapers and backgrounds designed for download for free. We hope you enjoy our growing collection of HD images to make use of as a background or home screen for the smartphone or computer. Please contact us if you intend to publish a beautifull background on our site. Only the best wallpapers. Day-to-day improvements of new, brilliant, HD wallpapers for pc and phones.
A ton of brilliant Fda Covid Vaccine Guidance wallpapers so that you can get a hold of with regard to free. You may also publish and promote your preferred wallpapers. HD wallpapers in addition to history images
Fda To Revise Pfizer S Covid 19 Vaccine Guidance After Allergic Reactions The Times Of Israel

Usa Fda Issues Covid 19 Vaccine Eua Guidance After Clash With White House Ris World

Fda Issues Covid 19 Vaccine Guidance As White House Reportedly Relents Medcity News

Fda covid vaccine guidance
On december 20 2020 acip issued recommendations for the use of moderna covid 19 vaccine for the prevention of covid 19. On december 18 2020 the fda issued an emergency use authorization for the use of the moderna covid 19 vaccine external icon for use in individuals 18 years of age and older. Development and licensure of vaccines to prevent covid 19. Fda issued guidance regarding the scientific data and information that would support the issuance of an emergency use authorization for an investigational vaccine intended to prevent covid 19. On december 11 2020 the u s. Related information developing and manufacturing drugs. This guidance is intended to remain in effect for the duration. Fda is issuing this guidance to provide sponsors of requests for emergency use authorization eua for covid 19 vaccines with recommendations regarding the data and information needed to support. Fda is issuing this guidance to assist sponsors in the clinical development and licensure of vaccines for the prevention of covid 19. The fda on tuesday released final guidance laying out its standards for approving coronavirus vaccines requiring that any vaccine candidate be at least 50 percent more effective than a placebo. On december 13 2020 the acip issued recommendations for the use of pfizer biontech s covid 19 vaccine for the prevention of covid 19. Fda is issuing this guidance to assist sponsors in the clinical development and licensure of vaccines for the prevention of covid 19.
Related post:


The Gruber Moment How Cber Got The Answers It Wanted On Covid Vaccine Guidance Pink Sheet

Us Fda Wants 2 Months Safety Data Before Approving Covid 19 Vaccine

Biopharma Industry Welcomes Fda Covid 19 Vaccine Guidance

Covid 19 Vaccines Advisory Committee Picks Apart Us Fda Guidance On Efficacy Endpoints Pink Sheet
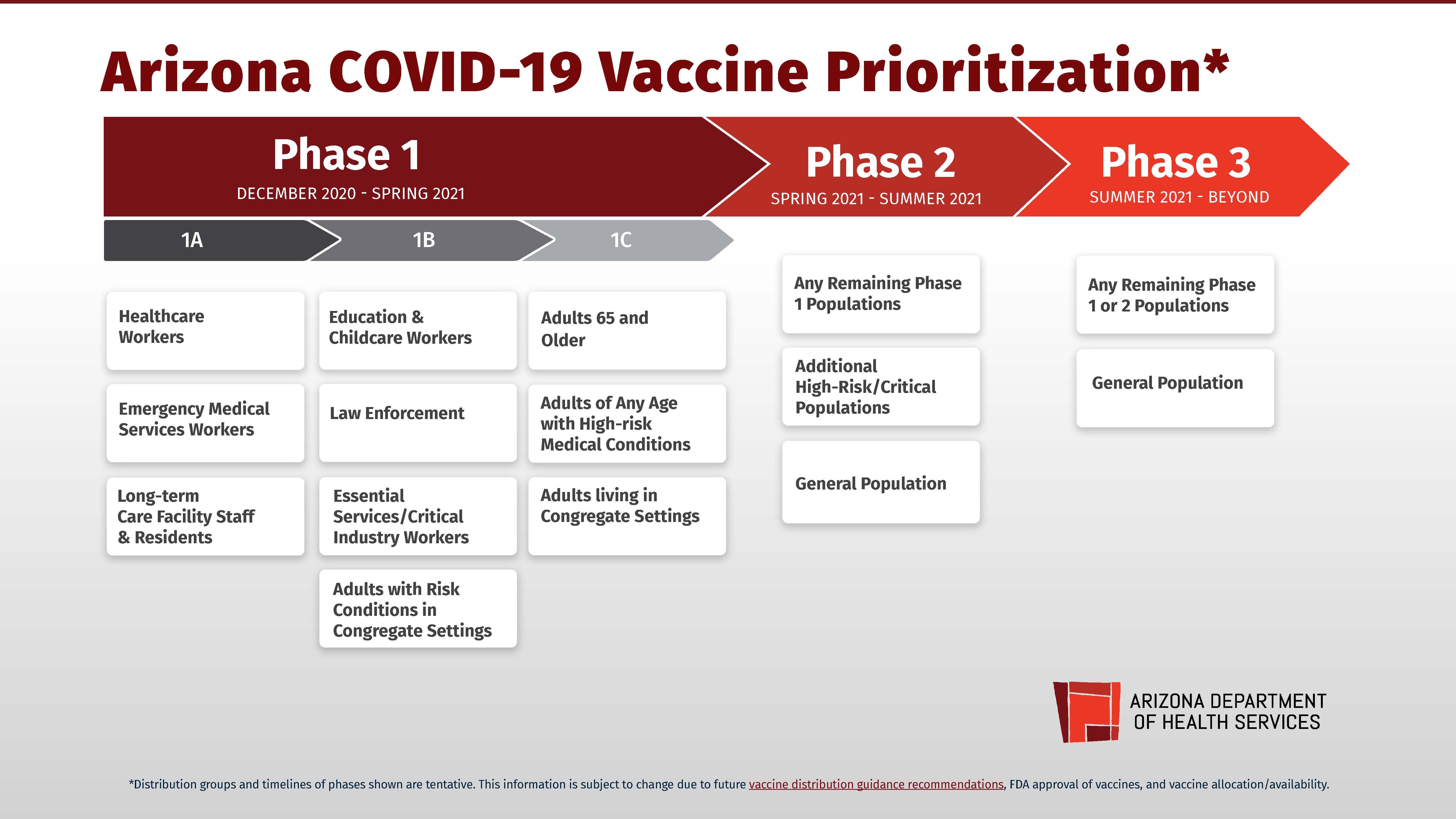
Vaccine Prioritization In Arizona Az Dept Of Health Services Director S Blog
Us Fda Safety Guidelines Likely To Push Covid 19 Vaccine Authorisation Past 2020 Election

Fda Staff Recommends Watching For Bell S Palsy In Moderna And Pfizer Vaccine Recipients
San Francisco Department Of Public Health

Covid Vaccine U S Plans To Ship 6 Million Moderna Doses Once Fda Gives Ok

Fda Guidance Provides Safety Quality Standards For Covid 19 Vaccine Development

Fda Sets Covid 19 Vaccine Safety Standards

Fda Establishes Clear Requirements For Covid 19 Vaccine Mofo Life Sciences

Fda Issues Eua For Pfizer Biontech Covid 19 Vaccine

Covid Vaccine Fda Will Scrutinize Allergic Reactions To Pfizer S Vaccine In Uk Before Clearing Use In U S

Fda Posts Vaccine Guidelines Blocked By White House

Us To Revise Covid Vaccine Guidance After Allergic Reactions World News Hindustan Times

Covid 19 Testing Fda Guidance Update Cortellis

Fda Issues Covid 19 Guidance Advice And Warnings
That's all about Fda Covid Vaccine Guidance, Fda is issuing this guidance to assist sponsors in the clinical development and licensure of vaccines for the prevention of covid 19. On december 13 2020 the acip issued recommendations for the use of pfizer biontech s covid 19 vaccine for the prevention of covid 19. The fda on tuesday released final guidance laying out its standards for approving coronavirus vaccines requiring that any vaccine candidate be at least 50 percent more effective than a placebo. Fda is issuing this guidance to assist sponsors in the clinical development and licensure of vaccines for the prevention of covid 19. Fda is issuing this guidance to provide sponsors of requests for emergency use authorization eua for covid 19 vaccines with recommendations regarding the data and information needed to support. This guidance is intended to remain in effect for the duration.



