Coffee Cup Calorimeter Formula

An accumulation of the most truly effective Coffee Cup Calorimeter Formula wallpapers and backgrounds readily available for download for free. Develop you enjoy our growing assortment of HD images to use as a background or home screen for the smartphone or computer. Please contact us if you intend to publish a awesome wallpaper on our site. Only the best wallpapers. Daily improvements of new, amazing, HD wallpapers for desktop and phones.
Plenty of wonderful Coffee Cup Calorimeter Formula wallpapers to be able to acquire for free. It is also possible to distribute and talk about your preferred wallpapers. HD wall papers along with qualifications illustrations or photos
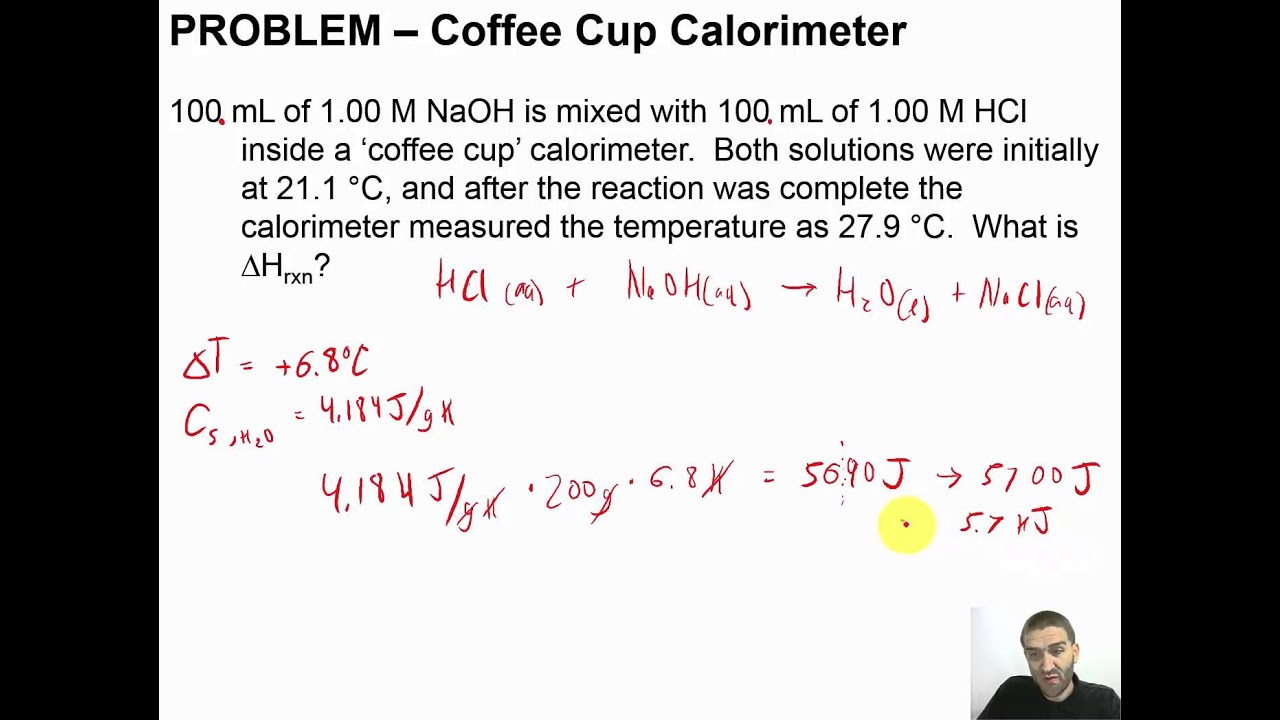
Chapter 09 17 Problem Coffee Cup Calorimeter Youtube
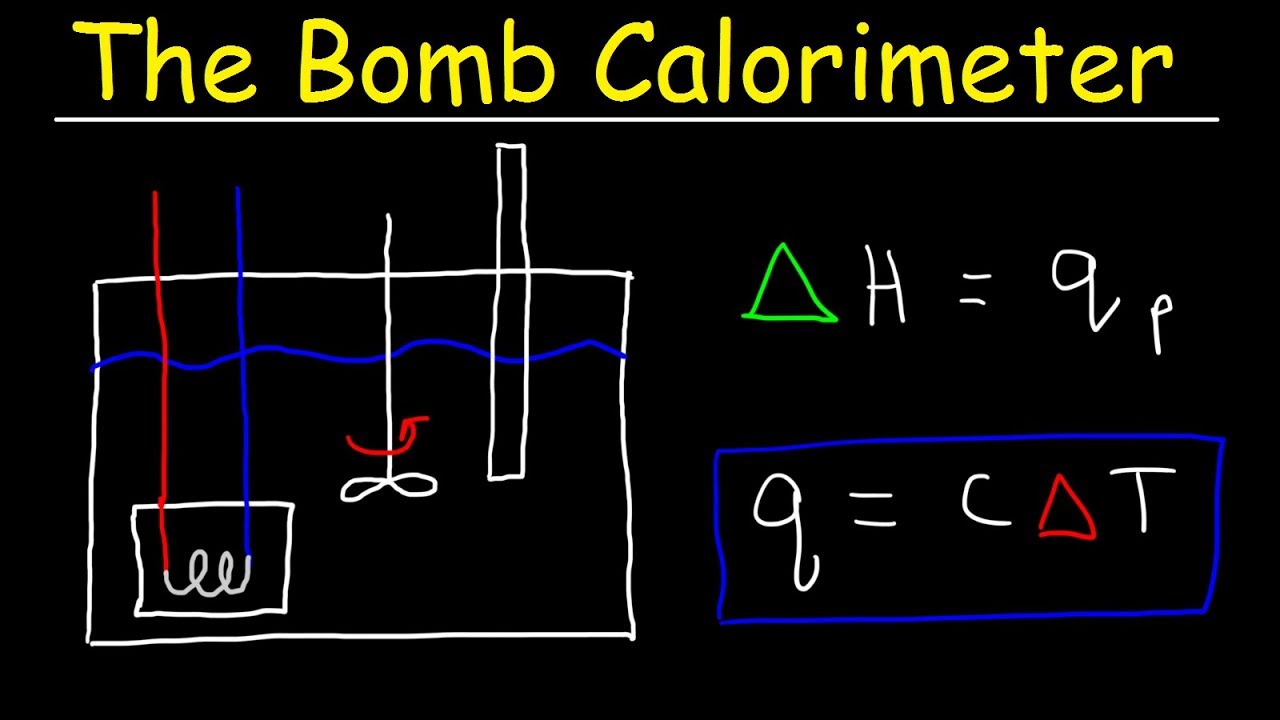
Bomb Calorimeter Vs Coffee Cup Calorimeter Problem Constant Pressure Vs Constant Volume Calorimet Youtube

Final Temperature Calorimetry Practice Problems Chemistry Youtube
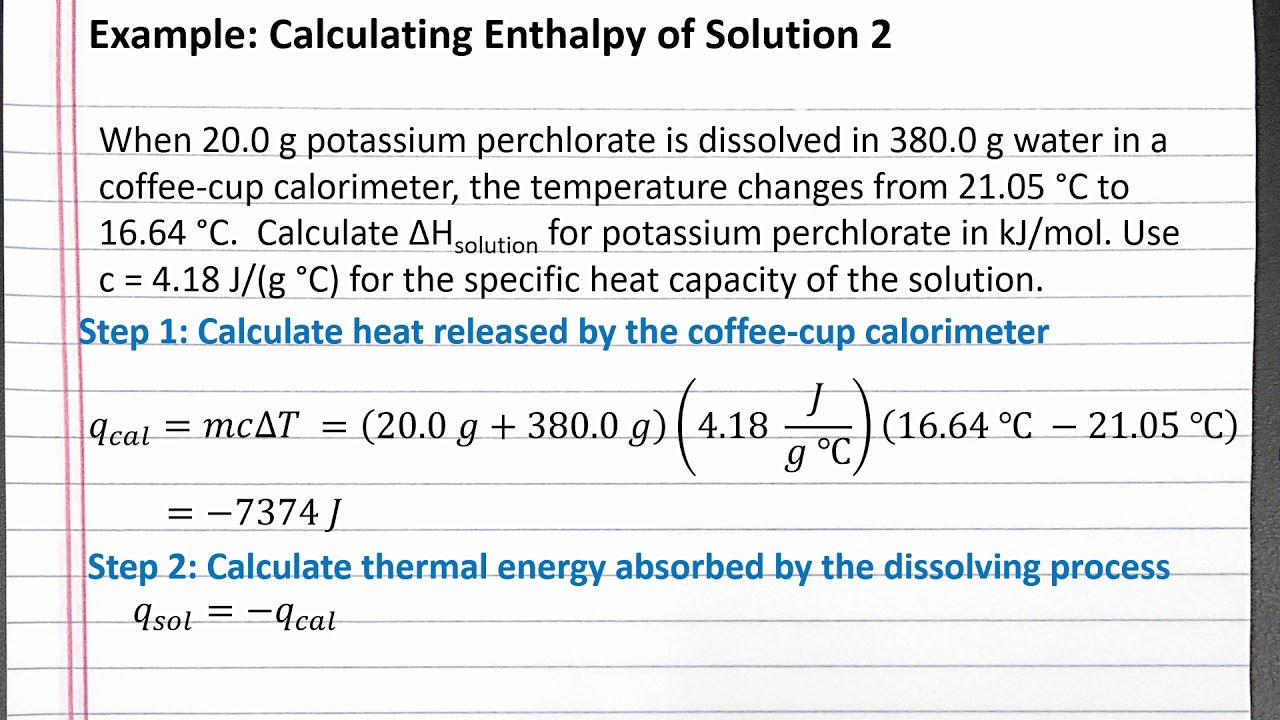
Coffee cup calorimeter formula
In this coffee cup calorimeter water is usually used as a solvent and a thermometer and if needed stirring stick are fitted snugly through small holes in the lid of the cup. It is defined as the amount of heat required to. Q calorimeter m c δt q calorimeter 100 0 g 4 18 j g c 18 1 c 35 4 c q calorimeter 7231 4 j. After mixing 100 0 g of water at 58 5 c with 100 0 g of water already in the calorimeter at 22 8 c the final temperature of the water is 39 7 c. The mass of the bomb multiplied by its specific heat is sometimes termed the calorimeter constant denoted by the symbol c with units of joules per degree celsius. 1557 7 j 24 9 c 62 6 j c. Q reaction q water q bomb where q water 4 18 j g c x m water x δt. 6 1 experiment 6 coffee cup calorimetry introduction. This chemistry video tutorial explains how to calculate the enthalpy change using a coffee cup calorimeter at constant pressure. The bomb has a fixed mass and specific heat. We will refer to the heat absorbed by the solution as q soln. A student wishes to determine the heat capacity of a coffee cup calorimeter. Here the calorimeter as in the q calorimeter term is considered to be the water in the coffee cup. Chemical reactions involve the release or consumption of energy usually in the form of heat heat is measured in the energy units joules j defined as 1 kg m2 s2. The heat is absorbed by the calorimeter assumed to be zero as discussed earlier and by the solution in the coffee cup calorimeter which consists of hcl aq and naoh aq at the beginning of the reaction and nacl aq and water at the end of the reaction.
Since the mass of this water and its temperature change are known the value of q calorimeter can be determined. Because they are equivalent scales delta t for celcius is the same as delta t for kelvin. Thus q ice m times c times delta t q ice 100 times 4 18 times 18 1 35 4 7231 4 j. Q neutr q soln. Q ice q calorimeter here the calorimeter is considered to be the water in the coffee cup. Also the mass of this water and its temperature change is known then the value can be determined.
Related post:


Chemistry 101 Constant Pressure Calorimetry Youtube

Solved Calorimetry Key Calculation Of Cal For The Coffe Chegg Com

Chem 101 Calculating Enthalpy Of Solution 2 Youtube

Solved In The Laboratory A Coffee Cup Calorimeter Or C Chegg Com

Coffee Cup Calorimetry Lab Study Com

Understanding Coffee Cup Calorimetry

Experiment 6 Coffee Cup Calorimetry

How To Solve Basic Calorimetry Problems In Chemistry Youtube

Chapter 6 Thermochemistry Chapter Ppt Download

Intro To Thermochem Discuss Heat V Temperature Ppt Video Online Download
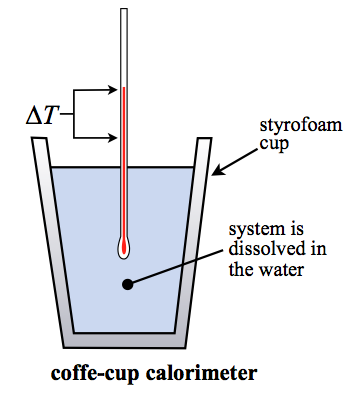
Coffee Cup Calorimetry

The Enthalpy Of Neutralization Of Phosphoric Acid Worksheet Studocu

Calorimetry Calorimetry Is Used To Measure Heat Capacity And Specific Heats Calorimeter An Instrument That Measures Heat Changes For Physical And Chemical Ppt Video Online Download

Solved 3 When 2 631 G Of Ax S Dissolves In 124 6 G Of Chegg Com

Solved When 2 690 G Of Ax S Dissolves In 148 6 G Of Wat Chegg Com

Final 1 Thermochemistry

Chemistry Coffee Cup Calorimetry Determine Delta H For Reaction Youtube

Internal Energy Heat And Work Chemistry Youtube
That's all about Coffee Cup Calorimeter Formula, Also the mass of this water and its temperature change is known then the value can be determined. Q ice q calorimeter here the calorimeter is considered to be the water in the coffee cup. Q neutr q soln. Thus q ice m times c times delta t q ice 100 times 4 18 times 18 1 35 4 7231 4 j. Because they are equivalent scales delta t for celcius is the same as delta t for kelvin. Since the mass of this water and its temperature change are known the value of q calorimeter can be determined.



